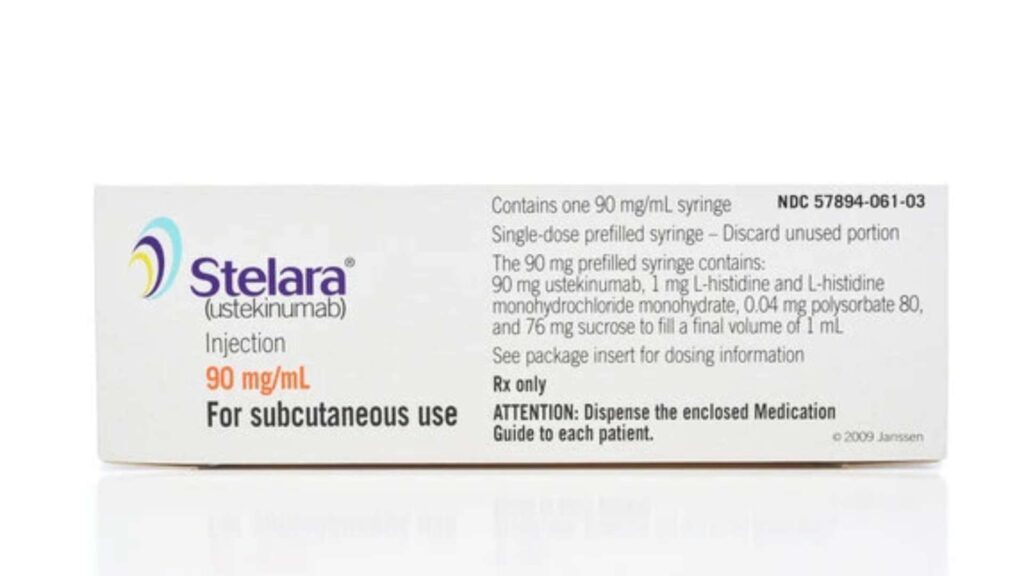
Stelara (Ustekinumab) is a biologic drug used to treat autoimmune inflammatory conditions. It is a fully human monoclonal antibody that targets inflammatory cytokines called interleukin-12 (IL-12) and interleukin-23 (IL-23). These Interleukins are overactive in certain autoimmune conditions, recruiting immune cells to the site of inflammation and promoting tissue damage. Stelara can block the activity of IL-12 and IL-23 to ease symptoms, manage disease progression, and improve overall quality of life.
How Does Blocking IL-12 and IL-23 Help in Autoimmune Diseases?
IL-12 and IL-23 proteins are key mediators for sustaining chronic inflammation in autoimmune diseases. Stelara inhibits the activity of these proteins to reduce chronic inflammation, ease the overactive immune response, and prevent flare-ups. Blocking the IL-12 and IL-23 has been proven effective in managing diseases like plaque psoriasis, ulcerative colitis, Crohn’s disease, and psoriatic arthritis.
Stelara Uses and Dosages
Managing autoimmune conditions is a challenging and complex process. Biologics like Stelara can help manage the disease progression and ease the debilitating symptoms. Stelara is FDA-approved for the following conditions:
Psoriatic Arthritis
Stelara reduces joint pain, swelling, and stiffness in patients with active psoriatic arthritis. During this condition, it can also help prevent joint structural changes (joint erosion, abnormal bone growth, etc.). Stelara can be used alone or in combination with methotrexate.
Stelara is generally prescribed as a 45 mg initial dose followed by a 45 mg booster dose at week 4 and then maintenance doses of 45 mg every 12 weeks. For patients with coexisting plaque psoriasis and body weight above 100 kg (220 lbs), the physician may prescribe a 90 mg dose. It is administered as a subcutaneous injection.
Plaque Psoriasis
Stelara effectively improves skin lesions, itching, and disease progression. It is prescribed for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Stelara is approved for use in adults and children (6 years or older) and is administered as a subcutaneous injection.
For moderate to severe plaque psoriasis, Stelara is generally prescribed as a 45 mg initial dose and a 45 mg maintenance dose every 12 weeks starting at week 4 from the initial dose. For adults weighing above 100 kg (220 lbs), the physician may recommend a 90 mg dose. For children (12 years or older) weighing 60 kg (132 lbs) or less, a 0.75 mg/kg dose is recommended, as the physician prescribes.
Crohn’s Disease and Ulcerative Colitis
Stelara is prescribed to treat moderate to severe Crohn’s Disease and Ulcerative colitis. It can help maintain disease remission, reduce flare-ups, and alleviate inflammation. The first dose is often a high-potency intravenous injection, while follow-up maintenance doses are administered subcutaneously.
Stelara is prescribed for adults and children 12 years of age or older. The recommended first dose for patients weighing 55 kg (121 lbs) or below is 260 mg (2 vials of 130 mg), injected intravenously. Patients weighing above 55 kg and below 85 kg (187 lbs) are generally prescribed 390 mg (3 vials of 130 mg) of the first intravenous dose. The recommended first dose for patients weighing above 85 kg is 520 mg (4 vials of 130 mg) of intravenous Stelara. The maintenance doses are 90 mg subcutaneous injections every 8 weeks, starting from the 8th week of the first dose.
Risks and Side Effects
Common side effects that come with Stelara include:
- Mild allergic reaction
- Headache
- Upper respiratory tract infections (for example, cold or flu like symptoms)
- Itching or swelling at the injection site
Serious but less common risks and side effects may include:
- Severe allergic reaction
- Serious infections such as tuberculosis (due to reactivation), hepatitis B, vaginal infections, and urinary tract infections
- Increased risk of certain cancers (e.g., skin cancer)
To prevent the reactivation of latent infections such as tuberculosis and hepatitis B, a preliminary screening is recommended before starting the therapy. Live vaccines should not be administered while on Stelara medication. Consult your physician if you plan to receive any vaccines.
Benefits of Stelara
- Stelara significantly improves patients’ quality of life suffering from autoimmune conditions.
- It can ease symptoms by reducing inflammation and alleviating pain as early as the third week after the first dose for treating ulcerative colitis, plaque psoriasis, and Crohn’s disease. Generally, the physician will evaluate the symptoms after the 16th week of starting treatment to assess if the disease is responding to Stelara.
- Stelara is convenient to use as it requires less frequent administration. The recommended interval between doses is 8 weeks, which can be extended depending on the severity of the symptoms (after consulting with your physician).
- Targeting the inflammatory cytokines can help manage the disease progression, prevent flare-ups, and induce remission.
Biosimilar of Stelara
The US FDA has approved several biosimilar biologics for Stelara, which are expected to be available for autoimmune patients in February 2025. Biosimilars are cost-effective alternatives that provide similar pharmacokinetics, efficacy, and safety to the generic drug ( Stelara). FDA has approved the following Stelara biosimilars as of November 2024:
- Wezlana (ustekinumab-auub) was jointly developed by Dong-A Socio Holdings and Meiji Seika Pharma. It is the first FDA-approved interchangeable biosimilar that can be substituted for a Stelara prescription at pharmacies without the prescriber’s intervention. It is approved for treating plaque psoriasis, ulcerative colitis, Crohn’s disease, and psoriatic arthritis. It was approved in November 2023 and is expected to be marketed in February 2025.
- Selarsdi (ustekinumab-aekn) is manufactured by Alvotech and Teva Pharmaceuticals and was approved in April 2024 for treating plaque psoriasis and psoriatic arthritis.
- Pyzchiva (ustekinumab-ttwe) is developed by Samsung Bioepis and marketed by Sandoz. It was approved in July 2024 and is expected to be marketed in February 2025.
- Otulfi (ustekinumab-aauz) was developed by Formycon and Fresenius Kabi. The FDA and European Commission approved it in September 2024 for treating plaque psoriasis, ulcerative colitis, Crohn’s disease, and psoriatic arthritis. A press release states it will be launched “no later than February 2025.”
- Imuldosa (ustekinumab-srlf) is developed by Dong-A ST and Meiji Seika Pharma. It was FDA-approved in October 2024 for treating plaque psoriasis, ulcerative colitis, Crohn’s disease, and psoriatic arthritis. It will be available on the market in the first half of 2025.
Stelara is manufactured by Johnson & Johnson, and its listed price is $25,497.12 for a 90 mg dose. Biosimilar biologics can cut the cost of generic biologics by 15% to 45% (up to 80% in some European and Asian markets), providing patients with more treatment options at affordable prices. Biosimilars are clinically similar to generic Stelara in efficacy, safety, pharmacokinetics, and side effects; however, they are not exact replicas of generic biologic medications.
Consult a healthcare professional to help select an appropriate biologic for autoimmune conditions.